missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Ravulizumab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
477.00 EUR - 1204.00 EUR
Specifications
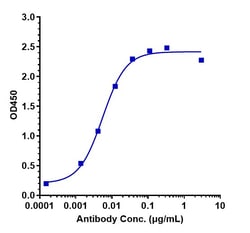
| Antigen | Ravulizumab Humanized |
|---|---|
| Concentration | 1 mg/mL |
| Content And Storage | -20°C, Avoid Freeze/Thaw Cycles |
| Applications | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Classification | Recombinant Monoclonal |
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
30283396
 |
Invitrogen™
MA559285 |
100 μg |
477.00 EUR
100µg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
|
30283728
|
Invitrogen™
MA559286 |
1 mg |
1204.00 EUR
1mg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Ravulizumab is considered a long-acting complement 5 (C5) inhibitor that has been undergoing clinical trials for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) as of 4 February, 2016. A pharmaceutical similar to ravulizumab (ALXN1210), called eculizumab, is currently approved for the treatment of PNH in 46 countries under the brand name Soliris™. Ravulizumab is considered by Alexion Pharmaceuticals Inc. to be a 'next-generation' eculizumab molecule. Ravulizumab was subsequently approved by the US FDA in December of 2018 for a variety of beneficial characteristics that make it an advanced, next-generation agent in comparison to eculizumab. In particular, ravulizumab is currently the first and only long-acting C5 complement inhibitor that can be administered every eight weeks for the treatment of adult patients with PNH whereas eculizumab is a bi-weekly treatment, Label. Moreover, virtually all of the phase 3 trial results for ravulizumab have demonstrated the equivalent efficacy and safety established by eculizumab and that patients transition safely and effectively from using eculizumab to ravulizumab. Subsequently, whereas PNH patients may have needed to previously plan their lives rather strictly around the bi-weekly infusion administrations of eculizumab, with ravulizumab such patients can find a more relaxed dosing schedule of only six or seven infusions over an entire year, Label.Specifications
| Ravulizumab Humanized | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Recombinant Monoclonal | |
| Lyophilized | |
| IgG2SA | |
| Human | |
| ALXN-1210; ravulizumab-cwvz | |
| Antibody |
| 1 mg/mL | |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| Unconjugated | |
| Human | |
| RUO | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Primary | |
| Protein A |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title