We have all heard much about PCR lately as this analysis technique has been under the spotlight because of its necessity in COVID-19 testing. This term, mostly reserved to scientists, specifically biologists, is now spoken of by many of us as if we all know exactly what it is. But what indeed is PCR? PCR stands for Polymerase chain reaction, a laboratory technique use to rapidly make copies of a segment of DNA. It allows scientists to take a very small fragment of DNA sequences, amplify it to a a large enough amount so they can study it in details in a second step.
For COVID-19 testing, the technique used is RT-PCR (Reverse Transcription Polymerase Chain Reaction): substances known as reverse transcriptase or DNA polymerase are added to a nasopharyngeal sample in a lab. Coronaviruses contain a non-segmented RNA genome. Reverse transcriptase is needed to transcribe viral RNA into cDNA (complementary DNA). The cDNA can subsequently be used as starting material in a PCR to amplify specific sequences from it using DNA polymerase. Specifically designed primers and probes attach themselves to sequences of cDNA to signal that a pathogen has been found.
This technique is used for quick medical diagnosis of diseases such as COVID-19, but also tuberculosis and other infections, and has also become commonplace in biomedical research and criminal forensics. But there is not one single PCR method, you will find below a table that explains the key advantages and disadvantages of each of them.
Real-time PCR vs. traditional PCR vs. digital PCR at a glance
| Digital PCR | Real-time PCR | Traditional PCR | |
|---|---|---|---|
| Overview | Measures the fraction of negative replicates to determine absolute copies | Measures PCR amplification as it occurs | Measures the amount of accumulated PCR product at the end of the PCR cycles |
| Quantitative? | Yes, the fraction of negative PCR reactions is fit to a Poisson statistical algorithm | Yes, because data is collected during the exponential growth (log) phase of PCR when the quantity of the PCR product is directly proportional to the amount of template nucleic acid | No, though comparing the intensity of the amplified band on a gel to standards of a known concentration can give you 'semi-quantitative' results |
| Applications |
|
| Amplification of DNA for:
|
| Summary | Advantages of digital PCR:
| Advantages of real-time PCR:
| Disadvantages of traditional PCR:
|
To understand why traditional PCR is limiting, it is important to understand what happens during a PCR reaction. A basic PCR run can be broken up into three phases:
- Exponential
Exact doubling of product is accumulating at every cycle (assuming 100% reaction efficiency). The reaction is very specific and precise. Exponential amplification occurs because all of the reagents are fresh and available, the kinetics of the reaction push the reaction to favour doubling of amplicon.
- Linear (high variability)
As the reaction progresses, some of the reagents are being consumed as a result of amplification. The reactions start to slow down and the PCR product is no longer being doubled at each cycle.
- Plateau (end-point: gel detection for traditional methods)
The reaction has stopped, no more products are being made and if left long enough, the PCR products will begin to degrade. Each tube or reaction will plateau at a different point, due to the different reaction kinetics for each sample. These differences can be seen in the plateau phase. The plateau phase is where traditional PCR takes its measurement, also known as end-point detection.
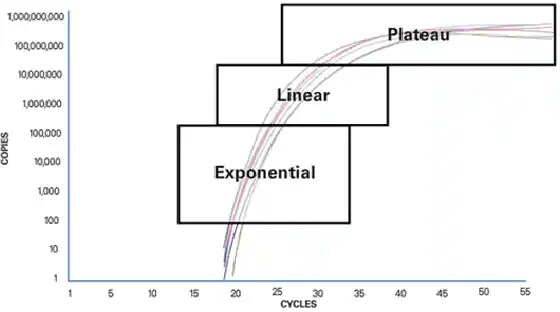
Figure 1: PCR phases.
Traditional PCR measures at the plateau, giving you variable results
In Figure 2, three replicate samples, which had same amount of DNA in the beginning of the reaction, have different quantities of PCR product by the plateau phase of the reaction (due to variations in reaction kinetics). Therefore, it will be more precise to take measurements during the exponential phase, where the replicate samples are amplifying exponentially.
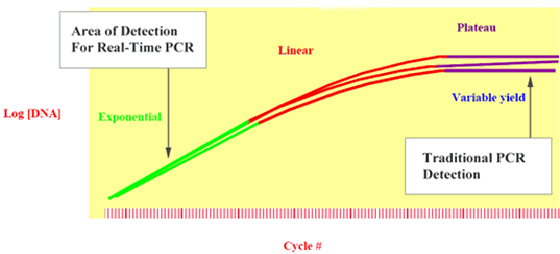
Figure 2: Identical samples produce different quantities of reaction product by the plateau phase of PCR.
Real-time PCR measures at the exponential phase for more accurate quantitation
Real-time PCR focuses on the exponential phase because it provides the most precise and accurate data for quantitation. Within the exponential phase, the real-time PCR instrument calculates two values. The Threshold Line is the level of detection at which a reaction reaches a fluorescent intensity above background. The PCR cycle at which the sample reaches this level is called the Cycle Threshold, Ct. The Ct value is used in downstream quantitation or presence/absence detection. By comparing the Ct values of samples of unknown concentration with a series of standards, the amount of template DNA in an unknown reaction can be accurately determined.
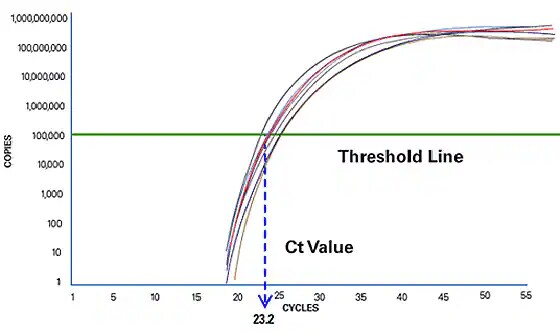
Figure 3: The PCR cycle at which the sample reaches a fluorescent intensity above background is the Cycle Threshold or Ct.
Digital PCR counts individual molecules for absolute quantification
Digital PCR works by partitioning a sample into many individual real-time PCR reactions; some portion of these reactions contain the target molecule (positive) while others do not (negative). Following PCR analysis, the fraction of negative answers is used to generate an absolute answer for the exact number of target molecules in the sample, without reference to standards or endogenous controls.
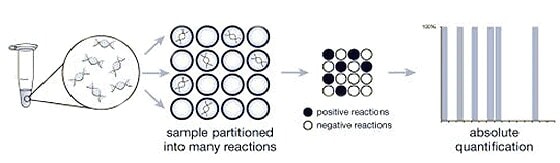
Figure 4: Digital PCR uses the ratio of positive (black) to negative (white) PCR reactions to count the number of target molecules.
