GMP Annex 1 - Are You Dressed For The Part?
What is EU GMP Annex 1?
Annex 1 is a legally binding part of EU GMP (European Union Good Manufacturing Practice) that provides guidelines and information relating to the manufacture of sterile medicinal products.
The new version of Annex 1 was published on 25th August 2022 and will be implemented one year later, on 25th August 2023.
What Does Annex 1 Cover?
The GMP Annex 1 provides comprehensive guidance on the design, construction, and maintenance of facilities and equipment used in the manufacture of medicinal products. It also includes guidelines on the production process, quality control, and documentation.
Annex 1 applies to all sterile medicinal products manufactured in the European Union and the UK, as well as those manufactured elsewhere and exported into the European Union and the UK. It applies to:
- Finished products
- Active substances
- Packaging materials
- Products provided in any size and combination
- Any manufacturing processes
- Any manufacturing technologies
- Any manufacturing scale, where the objective is to provide a sterile product
- The design and control of facilities, equipment, systems, and procedures
What Are the Biggest Contamination Risks During Aseptic Processing?
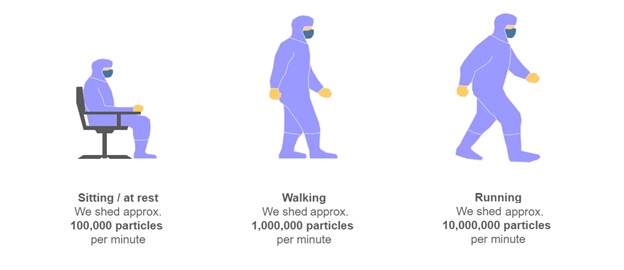
Sources of contamination within a cleanroom include raw materials, packaging, equipment, fluids, tools, processes and the most significant source of contamination... people. Microorganisms are shed from hair, skin, eyes and mucus membranes1. When people move around in controlled and sterile environment, they shed 10 times more particles than when they are sitting or at rest, hence the reason for clear guidelines on the correct controlled behavior of personnel in Annex 1 Part 7.18:
“Activities in clean areas that are not critical to the production processes should be kept to a minimum, especially when aseptic operations are in progress. Movement of personnel should be slow, controlled and methodical to avoid excessive shedding of particles and organisms due to over-vigorous activity. Operators performing aseptic operations should adhere to aseptic technique at all times to prevent changes in air currents that introduce air of lower quality into the critical zone. Movement adjacent to the critical zone should be restricted and the obstruction of the path of the unidirectional (first air) airflow should be avoided. A review of airflow visualisation studies should be considered as part of the training programme.”
Cleanroom Garments: Material and Construction
Part 7 of Annex 1 outlines the requirements needed regarding personnel numbers, behaviors, skills and protective clothing. A precise contamination control strategy needs to ensure that the personal protective equipment (PPE) they’re wearing is suitably assessed and monitored.
Outlined in the IEST-RP-CC003.4 standard - Garment system consideration for cleanrooms and other controlled environments, lists six types of non-woven fabrics for use in cleanrooms and other controlled and sterile environments, and describes each fabric as follows:
- Spunbonded or thermal bond - A non-woven fabric typically made from polypropylene in a relatively open structure. This type of non-woven fabric is more commonly used in bouffant caps, shoe covers, etc., it also does not demonstrate high barrier performance
- Flash spun - A non-woven fabric made of high-density polyethylene continuous fibres. Flash spun non-wovens have some barrier properties and are splash-resistant to water
- Melt blown – A material made from continuous polypropylene microfibres and used in composite structures of many types of face masks because of its high filtration efficiency and repellence. Melt blown fabric does not have adequate strength to be used alone for garments
- Spunbonded/melt blown/spunbonded (SMS) - A laminate structure made from polypropylene continuous fibres, SMS offer barrier protection and comfort
- Film laminate - A spunbonded layer laminated to non-porous films. Demonstrates particle, blood and chemical barrier properties but lacks air and moisture permeability
- Microporous film laminate - A laminate made from a spunbonded layer and a microporous film for improved barrier properties. This laminate is splash-resistant and has a blood barrier. Microporous film laminate is optimal for use in surgical areas and critical environments
When selecting garments for cleanroom use, depending on the specific application, the IEST standard recommends evaluating the fabric properties including testing for (selecting those relevant to the fabric type):
- Cleanliness and cleanability
- Electrostatic properties
- Biological properties
- Durability
- Comfort
- Opacity
- Particle filtration efficiency
- Microbial penetration
- Chemical compatibility
- Fluid resistance
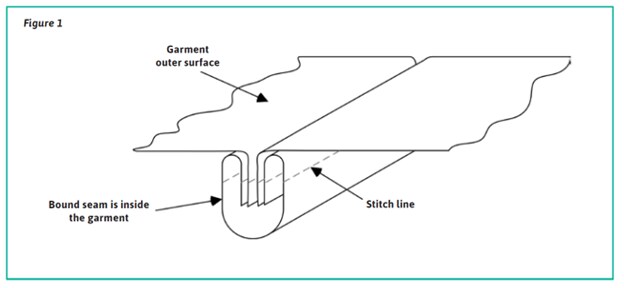
Construction of cleanroom garments is another important consideration, and the IEST standard outlines recommendations for thread and seam structure. Seams for cleanroom garments should be joining seams to avoid free-air/particulate passage from the inside of the garment to the outside environment. The IEST standard recommends for the construction of cleanroom garments that they are constructed using a bound joining seam, as shown in figure 1.
Personnel: Annex 1, Part 7 Guidance on Cleanroom Garments, Protective Clothing and Donning Procedures
As well as consideration for how the cleanroom garments are constructed and the type of material used, Annex 1 Parts 7.11 and 7.12 outline what garments to wear in each cleanliness grade, and how they should be worn and donned has been outlined in Part 7.13 and 7.14. Utilising cleanroom garments that are in compliance with industry standards offers better protection.
Protective Clothing and Quality
Annex 1 Part 7.11 states that protective clothing and its quality should be appropriate for the process and the grade of the working area. It should be worn in such a way as to protect the product from contamination. When the type of clothing chosen needs to provide the operator protection from the product, it should not compromise the protection of the product from contamination. Cleanroom garments should be visually checked for cleanliness and integrity immediately prior to and after gowning. Gown integrity should also be checked upon exit. For sterilised garments and eye coverings, particular attention should be taken to ensure they have been subject to the sterilisation process, are within their specified hold time and that the packaging is visually inspected to ensure it is integral before use. Reusable garments (including eye coverings) should be replaced if damage is identified, or at a set frequency that is determined during qualification studies. The qualification of garments should consider any necessary garment testing requirements, including damage to garments that may not be identified by visual inspection alone.
Limiting Shedding
Annex 1 Part 7.12 states that protective clothing should be chosen to limit shedding due to operators’ movement. This is important to minimize the risk of contamination from fibres or particles shed from the clothing.
Cleanliness Grades
A description of typical clothing required for each cleanliness grade is given below:
- Grade B (including access/interventions into grade A): appropriate garments that are dedicated for use under a sterilised suit should be worn before gowning (see paragraph 7.14). Appropriately sterilised, non-powdered, rubber or plastic gloves should be worn while donning the sterilised garments. Sterile headgear should enclose all hair (including facial hair) and where separate from the rest of the gown, it should be tucked into the neck of the sterile suit. A sterile facemask and sterile eye coverings (e.g. goggles) should be worn to cover and enclose all facial skin and prevent the shedding of droplets and particles. Appropriate sterilised footwear (e.g. overboots) should be worn. Trouser legs should be tucked inside the footwear. Garment sleeves should be tucked into a second pair of sterile gloves worn over the pair worn while donning the gown. The protective clothing should minimise shedding of fibres or particles and retain particles shed by the body. The particle shedding and the particle retention efficiencies of the garments should be assessed during the garment qualification. Garments should be packed and folded in such a way as to allow operators to don the gown without contacting the outer surface of the garment and to prevent the garment from touching the floor.
- Grade C: Hair, beards and moustaches should be covered. A single or two-piece trouser suit gathered at the wrists and with high neck and appropriately disinfected shoes or overshoes should be worn. They should minimise the shedding of fibres and particles.
- Grade D: Hair, beards and moustaches should be covered. A general protective suit and appropriately disinfected shoes or overshoes should be worn. Appropriate measures should be taken to avoid any ingress of contaminants from outside the clean area.
- Additional gowning including gloves and facemasks may be required in grade C and D areas when performing activities considered to be a contamination risk as defined by the CCS.
Donning Cleanroom Garments in Specific Grade Change Rooms (Part 7.14)
Cleanroom gowning should be performed in change rooms of an appropriate cleanliness grade to ensure gown cleanliness is maintained. Outdoor clothing including socks (other than personal underwear) should not be brought into changing rooms leading directly to grade B and C areas. Single or two-piece facility trouser suits, covering the full length of the arms and the legs, and facility socks covering the feet, should be worn before entry to change rooms for grades B and C. Facility suits and socks should not present a risk of contamination to the gowning area or processes.
Ansell Solutions: A Comprehensive Sterile PPE Portfolio for Annex 1 Compliance
Our extensive portfolio of controlled environment protection includes sterile cleanroom coveralls, gloves, goggles and facemasks to ensure you meet all the guidelines set out in Annex 1 Part 7.
GLOVES: Our sterile 300mm/12” gloves are available in Latex (NRL), Neoprene, Nitrile and PI and can be disinfected using alcohol disinfectants and double-donned to comply with aseptic gowning and SOPs. Our longer length 400mm/16” and 600mm/24” gloves provide extra coverage of the arm to make sure no risk of gaps between the garment and gloves.
COVERALLS (SUITS), HOODS AND OVERBOOTS: Our BioClean-D™ sterile disposable cleanroom coverall and accessories range includes sterile single-piece disposable suit with hood S-BDCHT, and S-BDSH drop-down garment, a coverall designed to be donned over the head for true aseptic donning or a disposable coverall without hood S-BDCCT, they all feature full length coverage of the arms and legs with elasticated wrists and ankles to ensure a secure fit. BioClean™ headgear comprising of 3-piece constructed hood S-BDHD-L, which features an extra-long cape to be tucked into the neck of the coverall to ensure no gaps for contamination risk. A range of BioClean™ standard and longer length sterile overboots S-BDOB and S-BDOB-L completes the offering.
COVERALL KITS: Our convenient coverall kits S-BDKM and S-BDHB include all the body protection a person needs in one package, coverall with collar (or hood), overboots, hood (with or without facemask), to reduce packaging waste and enable efficient donning with everything in one easy open package.
CHEMO PROTECTION RANGE: The BioClean-C™ range of aprons S-BCDA and S-BCAS and sleeve covers S-BCSC provide an extra layer of protection when handling chemotherapy drugs and have been tested against ASTM F739-12.
GOGGLES: Our range of BioClean™ goggles include a sterile single-use version BCGS1 and BVGS, autoclavable versions BCAG and BCAH, and another autoclavable version with a wraparound lens to provide panoramic vision BCAP. All BioClean™ goggles have anti-fog technology to ensure a clear view every time. They also feature indirect ventilation systems for wearer comfort while avoiding droplets and particles from entering the controlled environment.
FACEMASKS: The BioClean™ range of sterile product protection looped, tie-on, flat or duck-bill facemasks – MEA210-1, MTA210-1, BDBS, BDBS-G have high bacterial, particle and viral filtration efficiency ensuring your critical products stay protected from droplets and particles shed from operators.
ACCESSORIES: Also available is a range of BioClean™ bouffant caps S-BBC and beard snoods S-BBS to ensure all hair is covered.
What Is the Importance of Barrier Technologies?
Annex 1 places considerable emphasis on barrier technology to separate the operator from the product to maintain Grade A conditions. This can be accomplished using RABS and Isolator gloves:
Annex 1 Part 4.18 - RABS or Isolator gloves, which are different technologies, and the associated processes, should be designed to provide protection through separation of the grade A environment from the environment of the surrounding room. The hazards introduced from entry or removal of items during processing should be minimised and supported by high capability transfer technologies, or validated systems that robustly prevent contamination and are appropriate for the respective technology.
Annex 1 Part 4.21 - The materials used for glove systems (for both RABS and Isolator gloves), should be demonstrated to have appropriate mechanical and chemical resistance. The frequency of glove replacement should be defined within the CCS.
Ansell Solutions: Rabs and Isolator Gloves Portfolio
Our clean and sterile Nitrile RABS and Isolator glove range comprises gloves, mittens, sleeves and sleeve/glove systems. Gloves and mittens are 100% water leak-tested for glove integrity and resistant to VHP, IPA and disinfectants for in situ sanitising.
Other RABS and Isolator glove materials available are CSM, NRL, Neoprene, EPDM and EPDM+. The most suitable material will be dependent on the applications and can be decontaminated by autoclaving, VHP or IPA.
Ansell PPE Packaging and Sterilisation: Safeguarding Cleanroom Production
PPE packaging and sterilisation play a crucial role in maintaining a cleanroom environment during production. Inadequate packaging and sterilisation can lead to contamination of the cleanroom, thereby compromising the quality and integrity of the final product. It is therefore essential to choose appropriate packaging and sterilisation methods that meet the standards set for cleanroom environments.
All Ansell sterile PPE is double or triple-bagged in durable plastic packaging to reduce contamination and includes sterilisation indicators to show the PPE has been sterilised to a sterility Assurance Level (SAL) 10-6.
Certificates of Irradiation (Gamma) or Certificates of Processing (EtO) per product lot number can be downloaded via our easy to use certificate tool on www.ansell.com/life-sciences/certificates to prove the PPE has been subjected to the full sterilisation process. All packaging clearly states expiry and manufacturing dates.
References
1 Brandes, R. Aseptic Processing: Qualification of Personnel, Mass & Peither AG-GMP Publishing, April 12, 2012.



